Click to see full answer.

Element Fluorine (F), Group 17, Atomic Number 9, p-block, Mass 18.998. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content. Hybrid Orbital Control in Carbon Alloys. With two fluorine atoms in the cluster, differences are. Fluorine-F atom structure. Wave theory-United nature theory. Tejman Chaim Henry Dr. Name: Fluorine Symbol: F Atomic Number: 9 Atomic Mass: 18.998404 amu Melting Point:-219.62 °C (53.530006 K, -363.31598 °F) Boiling Point:-188.14 °C (85.01 K, -306.652 °F) Number of Protons/Electrons: 9 Number of Neutrons: 10 Classification: Halogen.
Also, does fluorine gain or lose electrons?It can lose one of its electrons, making it an ion. It now has more positive protons than electrons so it has an overall positive charge. A fluorine atom will tend to gain, rather than lose, an electron. By gaining a negative electron, it has an overall negative charge.
Fluorine Atomic Symbol
Beside above, what happens when an atom gains an electron? However, if something happens to make an atom lose or gain an electron then the atom will no longer be neutral. An atom that gains or loses an electron becomes an ion. If it gains a negative electron, it becomes a negative ion. If it loses an electron it becomes a positive ion (see page 10 for more on ions).
Just so, how many electrons does fluorine gain or lose?
Fluorine Atom Diagram
Example 1: A fluorine atom can get a full valence shell by either gaining one more electron, or by losing seven electrons. The former requires the transfer of less electrons, so the fluorine atom will try to gain one electron first. Therefore, F− ions are more common than F7+ ions.
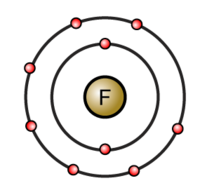
What happens when fluorine atoms react?
Fluorine is in Group 7. It has seven electrons in its outer shell. It gains an electron from another atom in reactions, forming a fluoride ion, F-. A fluoride ion has the same electronic structure as a neon atom (Ne).
