- Fluorine Valence Shell
- Fluorine Valence Electrons Number
- Fluorine Valence Bonds
- Fluorine Valence Electron Configuration
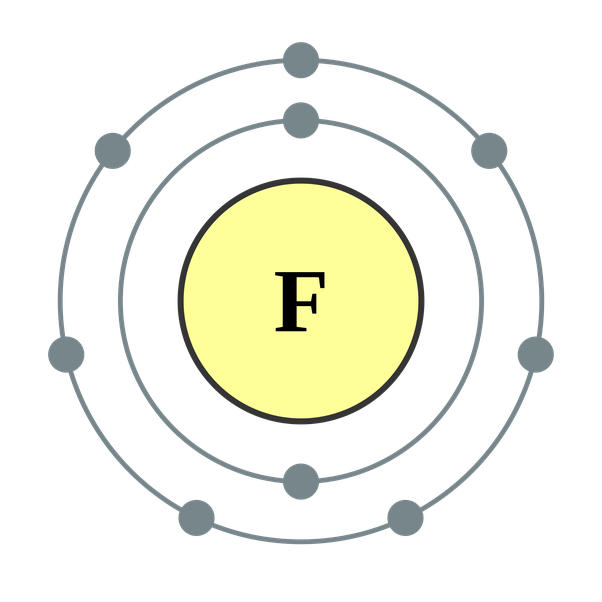
Since valence electrons are the ones which are contained in the outermost shell of the atom, Fluorine has 7 valence electrons. By each contributing one electron, they make the following molecule: In this molecule, the hydrogen atom does not have nonbonding electrons, while the fluorine atom has six nonbonding electrons (three lone electron pairs). The fluorine atom has seven valence electrons. The atomic number of fluorine is 9, and its electronic configuration is He 2s2 2p5. The atom of fluorine prefers to gain an electron to complete its last orbit. Fluorine forms a negative ion by gaining an electron during bond formation. In a fluorine atom, there is an unpaired electron in one of the 2p orbitals. When a F 2 molecule forms, the 2p orbitals from each of the two atoms overlap to produce the F−F covalent bond. The overlapping orbitals do not have to be of the same type. Find out the total number of valence electrons in PF3, which is 26. Find out the number of valence electrons further needed of a single PF3 molecule to stabilize itself. It is six in total, where three valence electrons are needed by the phosphorus atom and one, each by three fluorine atoms. The valency of fluorine is seven. This means that the outer electron shell of a fluorine atom contains seven electrons. An easy way to determine the. See full answer below.
Drawing the Lewis Structure for F2
Fluorine Valence Shell
Viewing Notes:
- F2 is a reddish gas at room temperature.
- The F2 Lewis structure is similar to Br2, Cl2, and I2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons.
- For the F2 Lewis structure there are a total of 14 valence electrons available.
Fluorine Valence Electrons Number

See the Big List of Lewis Structures
Transcript: OK, this is Dr. B. We're going to do the Lewis structure for F2, Fluorine gas: a yellow, extremely reactive gas. And we'll start looking on the periodic table. Fluorine is in group 7, or, sometimes called 17, and that means that it will have 7 valence electrons. But we have 2 Fluorine atoms, so we need to multiply that by 2, and that gives us a total of 14 valence electrons. Let's draw the two Fluorines next to each other. And we have 14 valence electrons to bond and then spread around and try to satisfy the octets, or give each Fluorine 8 valence electrons. We'll put these two here. That pair bonds them together.
And so we've used 2, 4, 6, 8, 10, 12, 14. We've used all our valence electrons up, and let's see if we have octets. Two, 4, 6, 8; that Fluorine has 8. And over here, 2, 4, 6, 8; that Fluorine has 8, as well. So we're actually done with the dot structure for F2. We could write it, though, like a structural formula, and that would look like this right here where this bond is represented by a single line. So that single line is a pair of electrons bonding the two F's together. And then the rest of the electrons are valence electrons around the Fluorine atom.
Fluorine Valence Bonds

Fluorine Valence Electron Configuration
This is Dr. B., and thanks for watching.
